EASIER
Gasification is most simply thought of as choked combustion or incomplete combustion.
It is burning solid fuels like wood or coal without enough air to complete combustion, so the output gas still has combustion potential. The unburned gas is then piped away to burn elsewhere as needed.
SMARTER
Gasification is a process to convert organic or fossil fuel based carbonaceous material into carbon monoxide, hydrogen and carbon dioxide.
This is achieved by reacting the material at high temperatures (>750 C), without combustion in combination with a controlled amount of oxygen or steam. The resulting gas mixture is called syngas and is a fuel. The power derived from gasification and combustion of the resultant gas is considered to be a source of renewable energy.
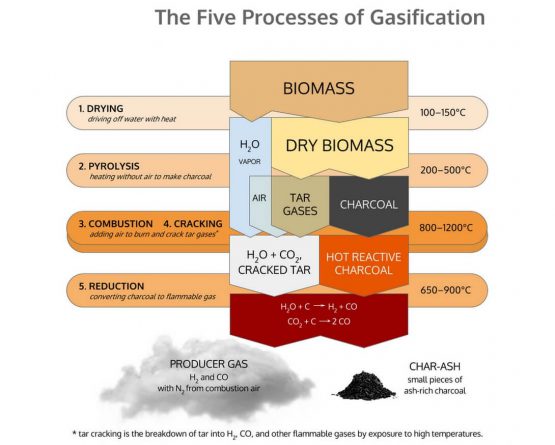
Gasification is made up for five discrete thermal processes: Drying, Pyrolysis, Combustion, Cracking, and Reduction. All of these processes are naturally present in the flame you see burning off a match, though they mix in a manner that renders them invisible to eyes not yet initiated into the mysteries of gasification. Gasification is merely the technology to pull apart and isolate these separate processes, so that we might interrupt the “fire” and pipe the resulting gases elsewhere.

BETTER
Renewable energy technologies are essential contributors to sustainable energy as they generally contribute to world energy security, reducing dependence on fossil fuel resources, and providing opportunities for mitigating greenhouse gases.
Conceptually, one can define three generations of renewables technologies, reaching back more than 100 years.
Biomass gasification technologies are considered Third-generation technologies.
System sections
Drying
The dehydration or drying process starts at around 100 °C. Typically the resulting steam is mixed into the gas flow and may be involved with subsequent chemical reactions, notably the water-gas reaction if the temperature is sufficiently high.
Drying is what removes the moisture in the biomass before it enters Pyrolysis. All the moisture needs to be (or will be) removed from the fuel before any above 100°C processes happen. All of the water in the biomass will get vaporized out of the fuel at some point in the higher temp processes. Where and how this happens is one of the major issues that has to be solved for successful gasification. High moisture content fuel, and/or poor handling of the moisture internally, is one of the most common reasons for failure to produce clean gas.
Pyrolysis
Pyrolysis is the process of heat to raw biomass, in an absence of air, so to break it down into charcoal and various tar gasses and liquids. It is essentially the process of charring.
Biomass begins to decompose with heat once its temperature rises above around 240°C. The biomass breaks down into a combination of solids, liquids and gasses. The solids that remain we commonly call charcoal. The gasses and liquids that are released we collectively call tars.
The input to gasification is some form of solid carbonaceous material— typically biomass or coal. All organic carbonaceous material is made up of carbon (C), hydrogen (H), an oxygen (O) atoms. The goal during the process is to break down the input into the simple fuel gasses of H2 and CO— hydrogen and carbon monoxide.
Combusting
The goal in combustion in a downdraft is to get good mixing and high temps so that all the tars are either burned or cracked, and thus will not be present in the outgoing gas. The char bed and reduction contribute a relatively little to the conversion of messy tars to useful fuel gasses. Solving the tar problem is mostly an issue of tar cracking in the combustion zone.
Cracking
Cracking is the process of breaking down large complex molecules such as tar into lighter gases by exposure to heat. This process is crucial for the production of clean gas that is compatible with an internal combustion engine because tar gases condense into sticky tar that will rapidly foul the valves of an engine. Cracking is also necessary to ensure proper combustion because complete combustion only occurs when combustible gases thoroughly mix with oxygen. In the course of combustion, the high temperatures produced decompose the large tar molecules that pass through the combustion zone.
Reduction
Reduction is the process stripping of oxygen atoms off combustion products of hydrocarbon (HC) molecules, so as to return the molecules to forms that can burn again. Reduction is the direct reverse process of combustion. Combustion is the combination of combustible gases with oxygen to release heat, producing water vapor and carbon dioxide as waste products. Reduction is the removal of oxygen from these waste products at high temperature to produce combustable gases. Combustion and Reduction are equal and opposite reactions. In fact, in most burning environments, they are both operating simultaneously, in some form of dynamic equilibrium, with repeated movement back and forth between the two processes.
Contact us
Would you like to discuss your project with our Experts? Please feel free to contact us.
Direct contact with our Experts
Your interest
Biomass Steam Reforming